Diols are important platform chemicals with broad industrial applications in biopolymer synthesis, cosmetics and fuels. The dependence of human production and life on non-renewable resources has led to increasingly serious depletion of fossil fuels and environmental problems. Using biomass including agricultural residues as raw materials, biosynthesis of diols has attracted worldwide attention due to its green and sustainable advantages.
Compared with other diols, ethylene glycol (EG) and 1,4-butanediol (BDO) lack natural anabolic pathways, hindering their large-scale commercial production. In recent years, with the development of systems metabolic engineering and synthetic biology methods, researchers can transform microbial chassis cells, which is expected to reduce the production cost of bio-based EG and BDO, and realize the transformation and replacement of petrochemical-based products. This paper summarizes the research progress of engineering microbial metabolic pathways for efficient biosynthesis of EG and BDO.
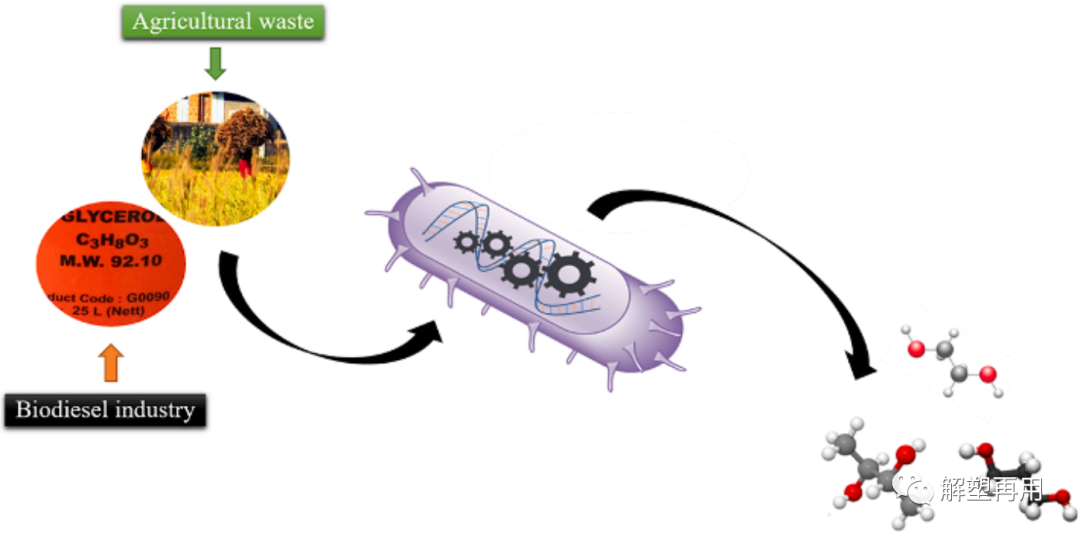
Synthetic microbial cell factory produces diols from inexpensive biomass resources
Metabolic pathway remodeling of ethylene glycol
Ethylene glycol (EG), a C2 diol, is an important chemical product mainly used in the manufacture of polyester fibers and polyethylene terephthalate (PET) resins. The international market for EG was estimated at $30 billion in 2020 and is expected to exceed $38 billion by 2027. The conventional production route of EG is through the hydrolysis of ethylene oxide and the dehydration of bioethanol (Jenkins, 2015; Zhang et al., 2013). Although the market application prospect is very broad, it is not economically feasible to directly manufacture EG from biomass at present. Microorganisms can metabolize glucose and other five-carbon sugars to EG.
The first attempt to generate EG from non-fossil xylose by Lui et al. knocked out the D-xylose isomerase gene in E. coli to block D-xylulose synthesis and enhance EG production (Liu et al., 2013 ). In this metabolic pathway, D-xylulose is converted to D-xylonic acid, which is further converted to 2-dehydro-3-deoxy-D-xylonate and 2-dehydro-3-deoxy-D- Xylonate, which is further broken down into glycolaldehyde and pyruvate. Finally, EG is formed from glycolaldehyde. Deletion of the xylose isomerase gene and overexpression of the glycolaldehyde reductase gene also increased EG production in E. coli (Cabulong et al., 2017).
.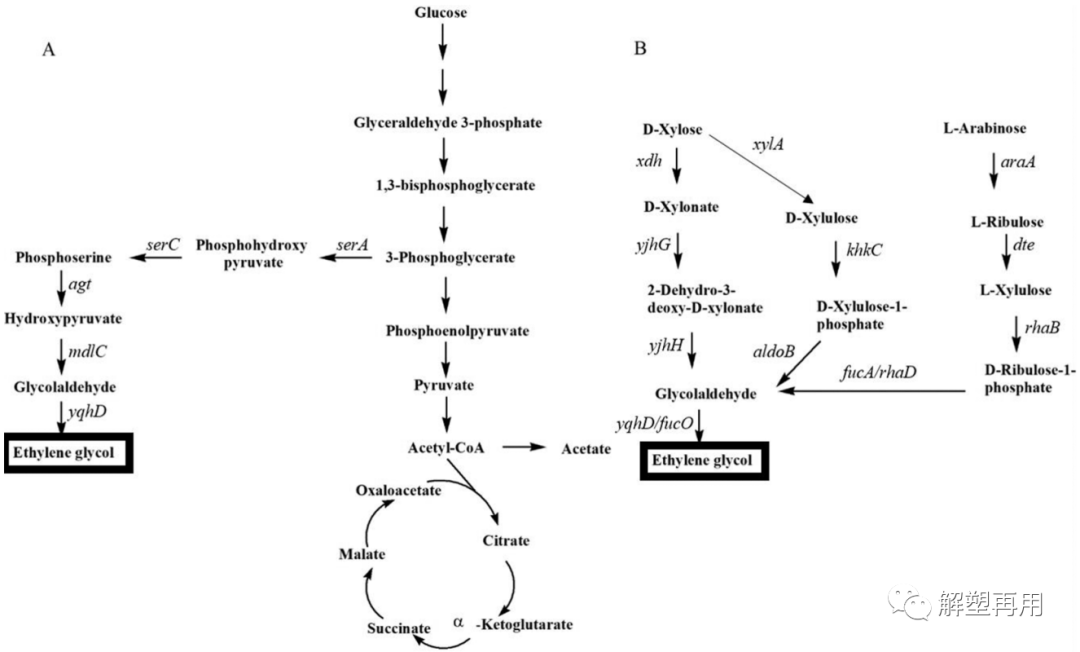
Ethylene glycol (EG) biosynthesis pathway
EG。The main disadvantage of the EG synthesis pathway using pentose as substrate is that only glycolaldehyde can be converted to EG, and the C3 intermediates pyruvate and dihydroxyacetone phosphate formed during pentose metabolism cannot be used for EG production (Zhang et al., 2017a , Zhang et al., 2017b). Chen et al. successfully transformed Corynebacterium glutamicum to realize the production of glucose to EG. Serine is formed from 3-phosphoglycerate, an intermediate in glycolysis, and can be converted to glycolaldehyde by two pathways. Serine can either be deaminated to form hydroxypyruvate or decarboxylated to form ethanolamine. Both compounds can be converted to glycolaldehyde, which can be further converted to EG.
Furthermore, EG synthesis was improved by overexpression of 3-phosphoglycerate dehydrogenase mutants, phosphoserine phosphatase and phosphoserine aminotransferase in C. glutamicum able to enhance serine synthesis (Peters-Wendisch et al., 2005). Although there have been some reports of bio-based EG synthesis, the current EG yield and production intensity are still low, and advances are needed to improve the production performance of engineered strains, making them more feasible than synthetic methods currently used in industry.
1,4-Butanediol Metabolic Pathway Remodeling
1,4-Butanediol (BDO) is a C4-compound mainly used in the production of polyester fibers and polyurethanes. The current industrial synthesis of BDO mainly adopts chemical methods. Since BDO is a highly reduced compound, no report of reducing the compound to generate terminal BDO has been found in any organism.
.Genomatica Company of the United States was the first to use Escherichia coli strains for BDO biosynthesis, and developed an artificial BDO synthesis pathway based on a genome-scale metabolic prediction model. Using succinyl CoA as a direct precursor, nearly 18 g/L of BDO was produced after 6 steps of catalysis. (Vivek et al., 2021). A synthetic pathway was established through a metabolic engineering strategy to utilize glucose for the microbial transformation of BDO (Fig. 3).
The process starts directly from succinyl-CoA in the TCA cycle and can be divided into an upstream pathway for the formation of the intermediate 4-hydroxybutyrate and a downstream pathway for the production of BDO from 4-hydroxybutyrate. This synthetic pathway is considered by many researchers to be the most promising route for microbial production of BDO, but it suffers from patent monopoly issues and involves lengthy enzymatic steps with complex metabolic regulation and low enzymatic kinetics. Formation will reduce the substrate conversion efficiency of BDO to some extent.
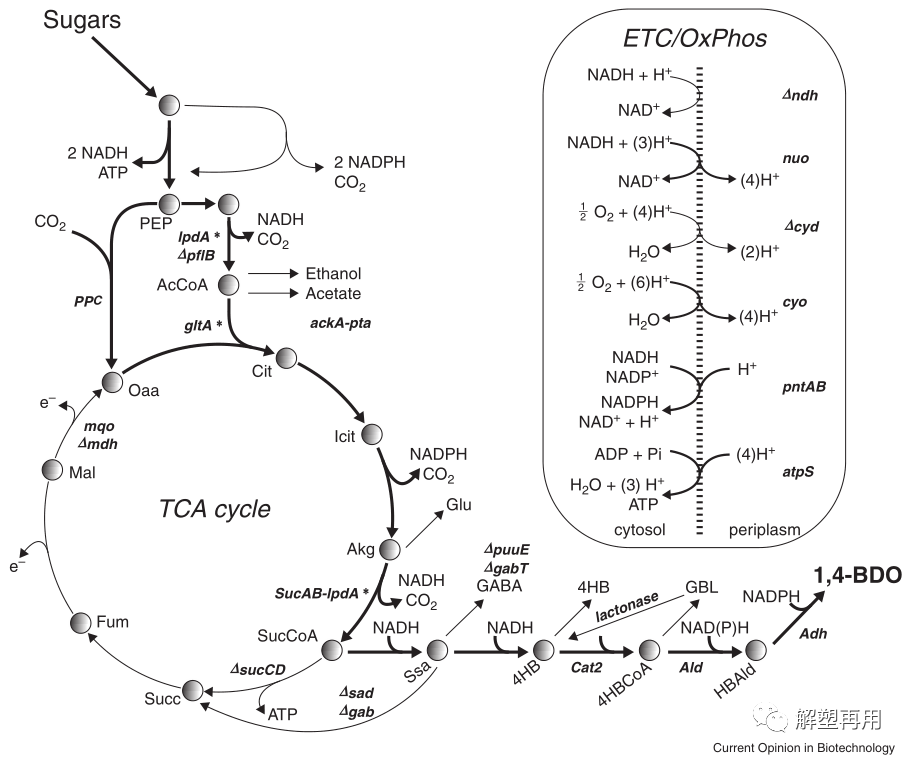
1,4-Butanediol (BDO) Biosynthesis Pathway
Liu and Lu in 2015 investigated a new pathway that exclusively utilizes xylose to produce BDO, in which xylose is converted into Alpha-ketoglutaric acid semialdehyde. Finally, α-ketoglutarate semialdehyde is converted to BDO through a reduction process in the presence of alcohol dehydrogenase, ketoacid decarboxylase (Liu et al., 2015). Recently, Wang and colleagues explored another artificial biosynthetic pathway using xylose as a carbon source.
Among them, xylose is selectively converted to 1,2,4-butanetriol first, and then diol dehydratase and aldolase convert 1,2,4-butanetriol to BDO. Increasing the enzymatic activity of diol dehydratase through rational protein engineering strategies can increase the accumulation of BDO, increasing the catalytic activity of the dehydratase towards 1,2,4-butanetriol by nearly 5-fold. A recombinant E. coli strain expressing the complete 1,4-BDO biosynthetic pathway produces 209 mg/L. Nonetheless, the dehydratase activity and xylose uptake rate need to be further improved before large-scale application (Wang et al., 2017).
Outlook
From the perspective of future development prospects, the biological production cost of bulk diols is expected to compete with industrialized processes. The innovative technology of biological synthesis of EG and BDO has received extensive attention in Europe and the United States and other countries, and has been put into industrial production . With the continuous support of the state in the fields of synthetic biotechnology and green biomanufacturing, it is believed that my country will make breakthroughs in the research and development of core biocatalysts in the near future, thereby supporting the large-scale commercial production of EG and BDO.
Post time: Jun-07-2022